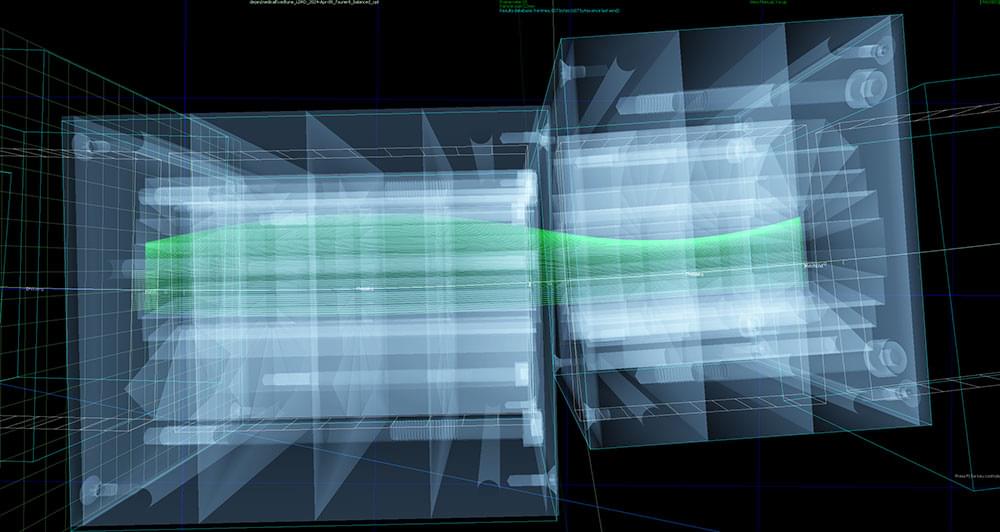
The slot-shaped aperture in the curved chain of magnets accommodates beams at different energies — a feature that would allow rapid switching among energies for more effective cancer treatment. This image shows a beam of light shining through the array with Mechanical Support Group staff in the background. (Kevin Coughlin/Brookhaven National Laboratory)
When the magnets arrived at Brookhaven, Katie Chen, a mechanical engineer, produced an architectural model of the assembly that Rob Karl, Adrian Timon, Travis Herbst, and Edward Dabrowski from the Mechanical Support Group used to properly align the magnets and bolt them to a supporting steel plate. To test that the magnets would accommodate the planned beam trajectories, the team transported the assembled array to the NASA Space Radiation Laboratory (NSRL), a facility that draws particles from the collider-accelerator complex supplying beams to Brookhaven Lab’s nuclear physics research facilities.
“This team tirelessly dedicated their time and expertise to completing the assembly and worked with exceptional dedication throughout Father’s Day weekend to help with these tests,” Mahler said.