I have a new essay out via the wonderful site Merion West. The article is based on some of my experimental writings at Oxford. I hope you’ll read and consider it. I’m highly worried life extension science isn’t moving forward fast enough!
“Sadly, biological humans are likely to be mortal for centuries more, unless a dramatic increase of both resources and life extension scientists are marshaled.”
Certain well-known gerontologists and longevity experts around the world believe that sometime in this century—probably in the next 15–50 years—medicine will likely overcome and cure most forms of disease, and even death itself. Billionaires such as Meta’s Mark Zuckerberg, Amazon’s Jeff Bezos, Alphabet’s Larry Page, and Oracle’s Larry Ellison have jumped on board, pledging billions of dollars to “conquering all disease by this century” and mortality altogether.
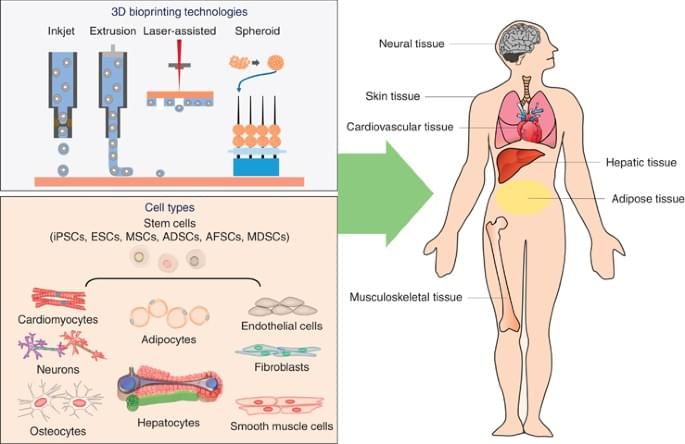
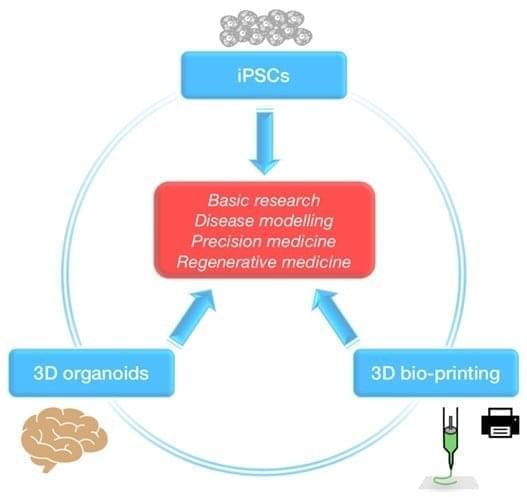
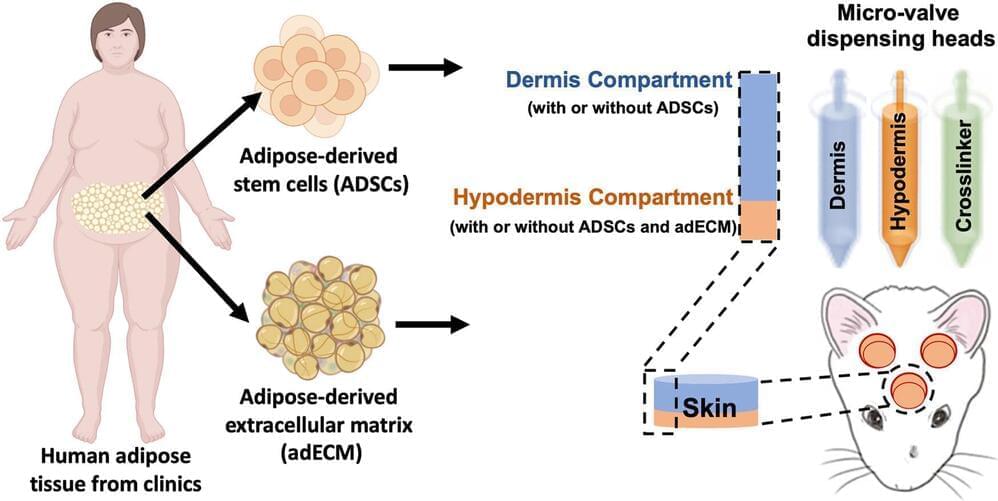
These business titans hope age reversal techniques via genetic editing therapies, stem cell rejuvenation, 3D bioprinting of organs, and the widespread creation of synthetic organs like artificial hearts could keep people indefinitely young and healthy. If biological human death from disease and aging are overcome, then only catastrophic accidental death—such as an airplane crash or incineration—can kill people. (Accidental death in this vein accounts for about seven percent of all deaths in the United States.)
Transhumanists believe that the human being is like a machine—an entity that can be fixed and made to overcome nearly all biological death. The question is how fast can this be done? If the human being is indeed a machine-like entity as nearly all credible scientists propose, then the answer almost certainly rests not in the limits of biology but, rather, in the amount of work and resources put into the life extension field.