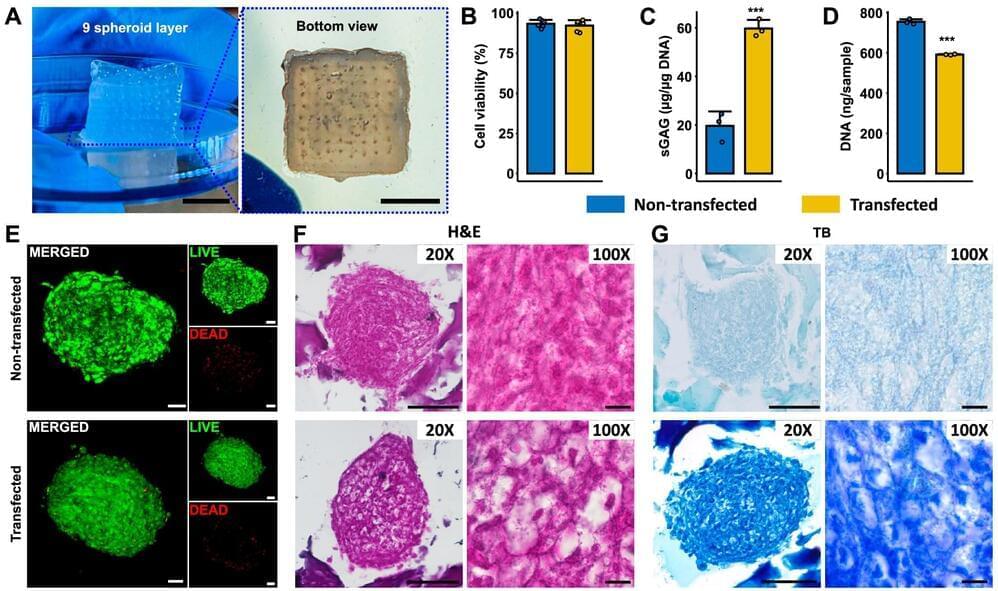
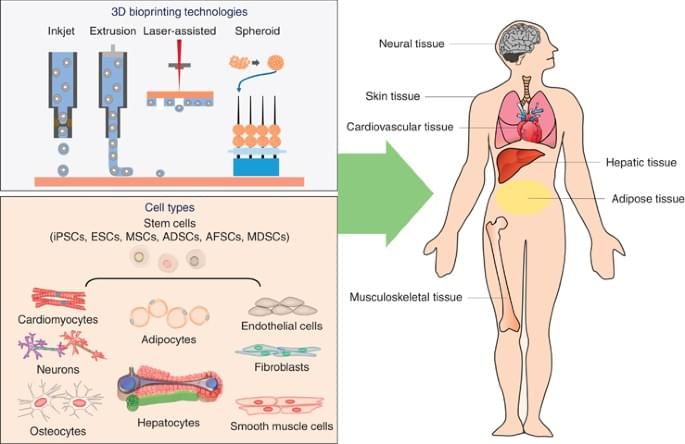
Three-dimensional (3D) printing isn’t just a way to produce material products quickly. It also offers researchers a way to develop replicas of human tissue that could be used to improve human health, such as building organs for transplantation, studying disease progression and screening new drugs. While researchers have made progress over the years, the field has been hampered by limited existing technologies unable to print tissues with high cell density at scale.
A team of researchers from Penn State have developed a novel bioprinting technique that uses spheroids, which are clusters of cells, to create complex tissue. This new technique improves the precision and scalability of tissue fabrication, producing tissue 10-times faster than existing methods. It further opens the door to developing functional tissues and organs and progress in the field of regenerative medicine, the researchers said.
They published their findings in Nature Communications.