4k words, 19 minutes reading time



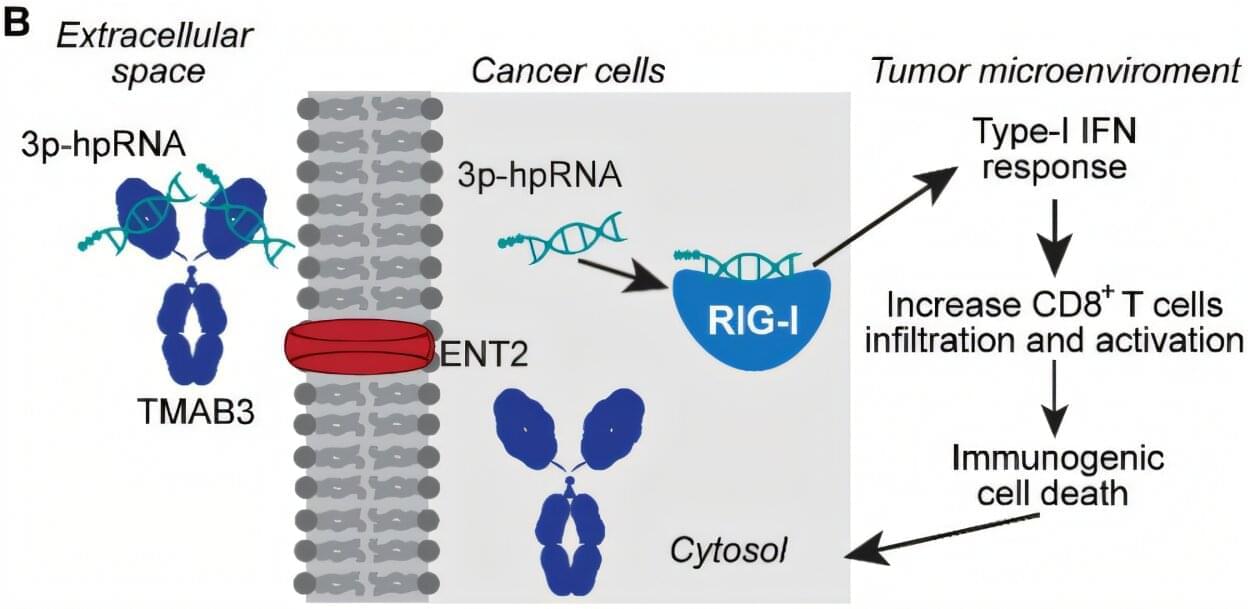
A specially engineered antibody that can accurately deliver RNA treatments into hard-to-reach and hard-to-treat tumors significantly improved survival and reduced tumor sizes in animal models, according to a study reported in Science Translational Medicine.
The study provides evidence that, once injected into the bloodstream, the antibody TMAB3, combined with a type of RNA that stimulates an innate immune reaction, can localize to tumors and penetrate and destroy stubborn diseased cells in pancreatic, brain, and skin cancers.
“Delivery of RNA-based therapies to tumors has been a challenge. Our finding that TMAB3 can form antibody/RNA complexes capable of delivering RNA payloads to tumors provides a new approach to overcome this challenge,” says Peter Glazer, senior author and Robert E. Hunter Professor of Therapeutic Radiology and Genetics at Yale School of Medicine (YSM).

IN A NUTSHELL 🌐 The SynHG project aims to synthesize a complete human genome, opening new horizons in biotechnology. ⚖️ Ethical considerations are central to the project, with a focus on responsible innovation and diverse cultural perspectives. 🧬 Initial steps involve creating a fully synthetic human chromosome, leveraging advances in synthetic biology and DNA chemistry.
This is a ~18 min talk plus ~10 min Q&A on a top-down approach to bioengineering and robotics that I gave at the Biohybrid Robotics Symposium in Switzerland in July 2025 (https://biohybrid-robotics.com/).
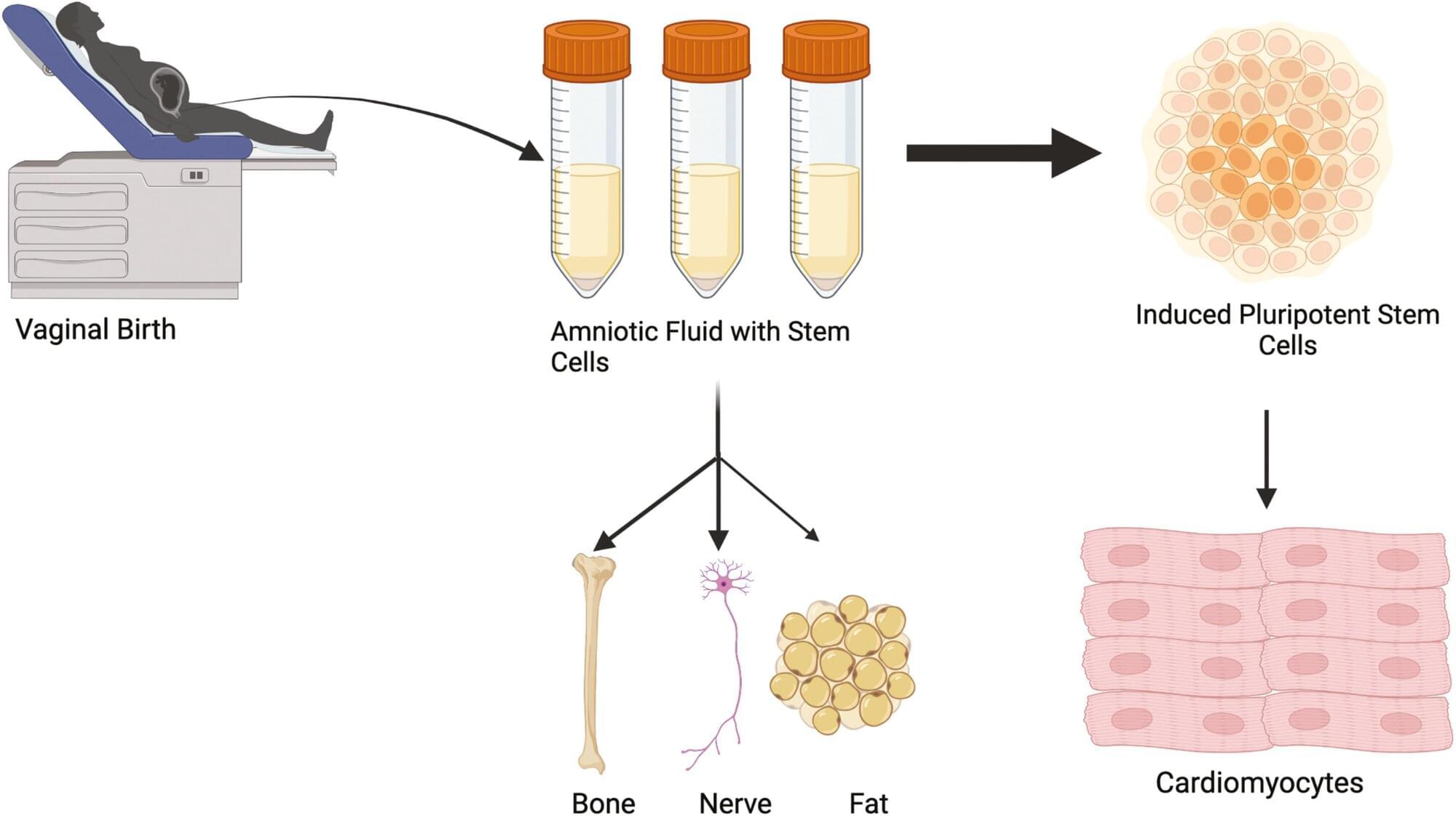
Researchers at the University of Colorado Anschutz Medical Campus have discovered that amniotic fluid stem cells can be safely collected from vaginal fluid after childbirth rather than relying on more invasive methods that can pose some risk to the mother and fetus.
“We can then turn those cells into beating heart cells and use them later in treating congenital heart defects,” said the study’s senior author Jeffrey Jacot, Ph.D., associate professor of pediatrics and bioengineering at the University of Colorado Center for Bioengineering in the CU School of Medicine. “These results allow for an expanded and readily available source of amniotic stem cells beyond traditional collection through amniocentesis.”
The study was published today in the journal Stem Cells Translational Medicine.
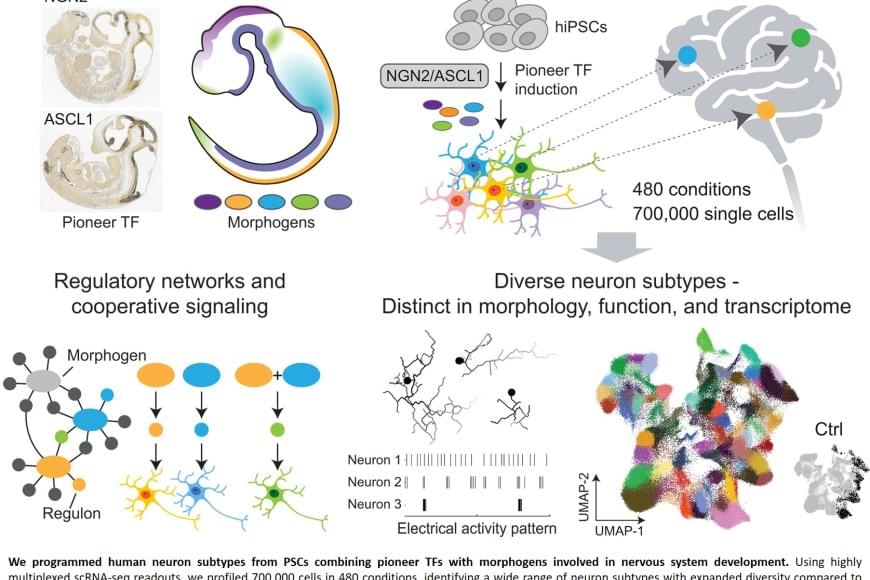
Nerve cells are not just nerve cells. Depending on how finely we distinguish, there are several hundred to several thousand different types of nerve cell in the human brain according to the latest calculations. These cell types vary in their function, in the number and length of their cellular appendages, and in their interconnections. They emit different neurotransmitters into our synapses and, depending on the region of the brain – for example, the cerebral cortex or the midbrain – different cell types are active.
When scientists produced nerve cells from stem cells in Petri dishes for their experiments in the past, it was not possible to take their vast diversity into account. Until now, researchers had only developed procedures for growing a few dozen different types of nerve cell in vitro. They achieved this using genetic engineering or by adding signalling molecules to activate particular cellular signalling pathways. However, they never got close to achieving the diversity of hundreds or thousands of different nerve cell types that actually exists.
“Neurons derived from stem cells are frequently used to study diseases. But up to now, researchers have often ignored which precise types of neuron they are working with,” saysthe senior author. However, this is not the best approach to such work. “If we want to develop cell culture models for diseases and disorders such as Alzheimer’s, Parkinson’s and depression, we need to take the specific type of nerve cell involved into consideration.”
IN A NUTSHELL 🌾 The Moon-Rice project is developing “super-dwarf” rice to support long-duration space missions and extreme Earth environments. 🛰️ Led by the Italian Space Agency, the project involves collaboration between three Italian universities specializing in rice genetics, crop physiology, and space crop production. 🔬 The research focuses on using CRISPR-Cas technology to create
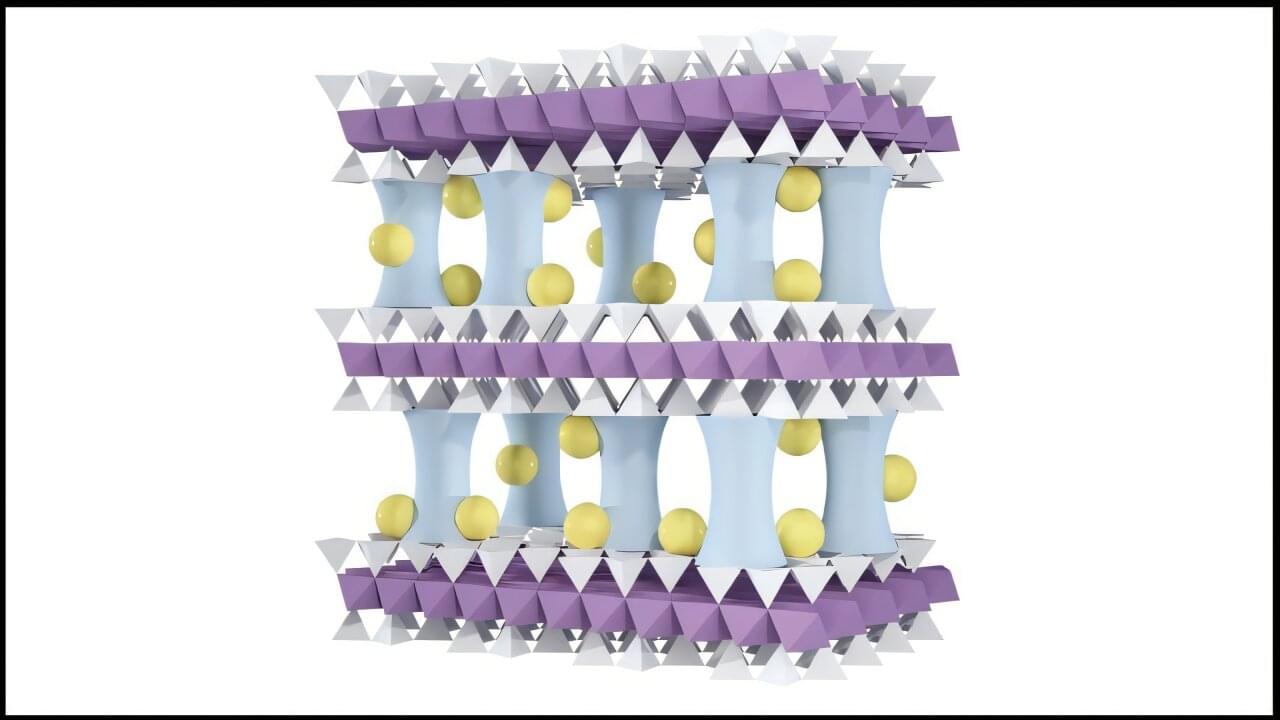
Lithium, the lightest metal on the periodic table, plays a pivotal role in modern life. Its low weight and high energy density make it ideal for electric vehicles, cellphones, laptops and military technologies where every ounce counts. As demand for lithium skyrockets, concerns about supply and reliability are growing.
To help meet surging demand and possible supply chain problems, scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have developed an innovative membrane technology that efficiently extracts lithium from water. Several team members also hold joint appointments with the Pritzker School of Molecular Engineering (PME) at the University of Chicago.
The findings appear in the journal Advanced Materials.