face_with_colon_three #Awesome
Get the latest international news and world events from around the world.
Experimental: Address correspondence to: Olga A
Guryanova, 1,200 Newell Drive, PO Box 100,267, Gainesville, Florida, 32,610, USA. Phone: 352.294.8590; Email: [email protected].
Find articles by Yan, B. in: | Google Scholar
1University of Florida College of Medicine, Gainesville, Florida, USA.

AI identifies key mpox protein for new vaccine and antibody therapies
With the help of artificial intelligence, an international team of researchers has made the first major inroad to date toward a new and more effective way to fight the monkeypox virus (MPXV), which causes a painful and sometimes deadly disease that can be especially dangerous for children, pregnant women and immunocompromised people.
Reporting in the journal Science Translational Medicine, the team found that when mice were injected with a viral surface protein recommended by AI, the animals produced antibodies that neutralized MPXV, suggesting the breakthrough could be used in a new mpox vaccine or antibody therapy.
In 2022, mpox began to spread around the world, causing flulike symptoms and painful rashes and lesions for more than 150,000 people, while causing almost 500 deaths. Vaccines developed to fight smallpox were repurposed amid the outbreak to help the most vulnerable patients, but that vaccine is complicated and costly, due to its manufacture from a whole, weakened virus.

Multiple modes of transcriptional regulation by the nuclear hormone receptor RARγ in human squamous cell carcinoma
Vitamin A metabolism and signaling through nuclear retinoic acid receptors (RARs α,β,γ) regulate embryogenesis, immune functions, and cell differentiation in most cell types. RARγ is highly expressed in stratified squamous epithelial cells of the oral cavity and skin. While data indicate that RARγ agonism is anti-tumorigenic in oral cavity squamous cell carcinoma (OCSCC), the specific, primary gene targets of RARγ remain poorly characterized. Here, we define RARγ signaling pathways through integrating genome-wide RARγ binding by Cleavage under Targets and Release Using Nuclease (CUT&RUN), chromatin histone marks, and global transcriptomics ± agonists in human OCSCC cells and in human OCSCC cells with deletion of RARG (RARGKO).


Short-Term Head-Out Whole-Body Cold-Water Immersion Facilitates Positive Affect and Increases Interaction between Large-Scale Brain Networks
An emerging body of evidence indicates that short-term immersion in cold water facilitates positive affect and reduces negative affect. However, the neural mechanisms underlying these effects remain largely unknown. For the first time, we employed functional magnetic resonance imaging (fMRI) to identify topological clusters of networks coupled with behavioural changes in positive and negative affect after a 5 min cold-water immersion. Perceived changes in positive affect were associated with feeling more active, alert, attentive, proud, and inspired, whilst changes in negative affect reflected reductions in distress and nervousness.
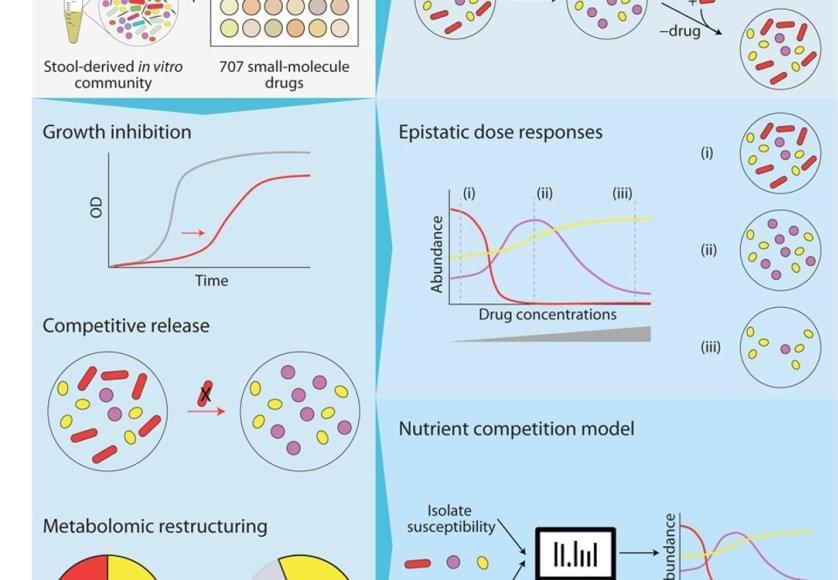
Medications change our gut microbiome in predictable ways
The bacteria in our poop are a reasonable representation of what’s living in our digestive system. To understand how different drugs can impact the gut microbiome, the team cultured microbial communities from nine donor fecal samples and systematically tested them with 707 different clinically relevant drugs.
The researchers examined changes in the growth of different bacterial species, the community composition, and the metabolome – the mix of small molecules called metabolites that microbes produce and consume. They found that 141 drugs altered the microbiome of the samples and even short-term treatments created enduring changes, entirely wiping out some microbial species. The primary force behind how the community responds to drug inhibition was competition over nutrients.
“The winners and losers among our gut bacteria can often be predicted by understanding how sensitive they are to the medications and how they compete for food,” said the first author on the paper. “In other words, drugs don’t just kill bacteria; they also reshuffle the ‘buffet’ in our gut, and that reshuffling shapes which bacteria win.”
Despite the complexity of the bacterial communities, the researchers were able to create data-driven computer models that accurately predicted how they would respond to a particular drug. They factored in the sensitivity of different bacterial species to that drug and the competitive landscape – essentially, who was competing with whom for which nutrients.
Their work provides a framework for predicting how a person’s microbial community might change with a given drug, and could help scientists find ways to prevent these changes or more easily restore a healthy gut microbiome in the future.
Our gut microbiome is made up of trillions of bacteria and other microbes living in our intestines. These help our bodies break down food, assist our immune system, send chemical signals to our brain, and potentially serve many other functions that researchers are still working to understand. When the microbiome is out of balance – with not enough helpful bacteria or the wrong combination of microbes – it can affect our whole body.
