Using CRISPR, an immune system bacteria use to protect themselves from viruses, scientists have harnessed the power to edit genetic information within cells. In fact, the first CRISPR-based therapeutic was recently approved by the FDA to treat sickle cell disease in December 2023. That therapy is based on a highly studied system known as the CRISPR-Cas9 genetic scissor.
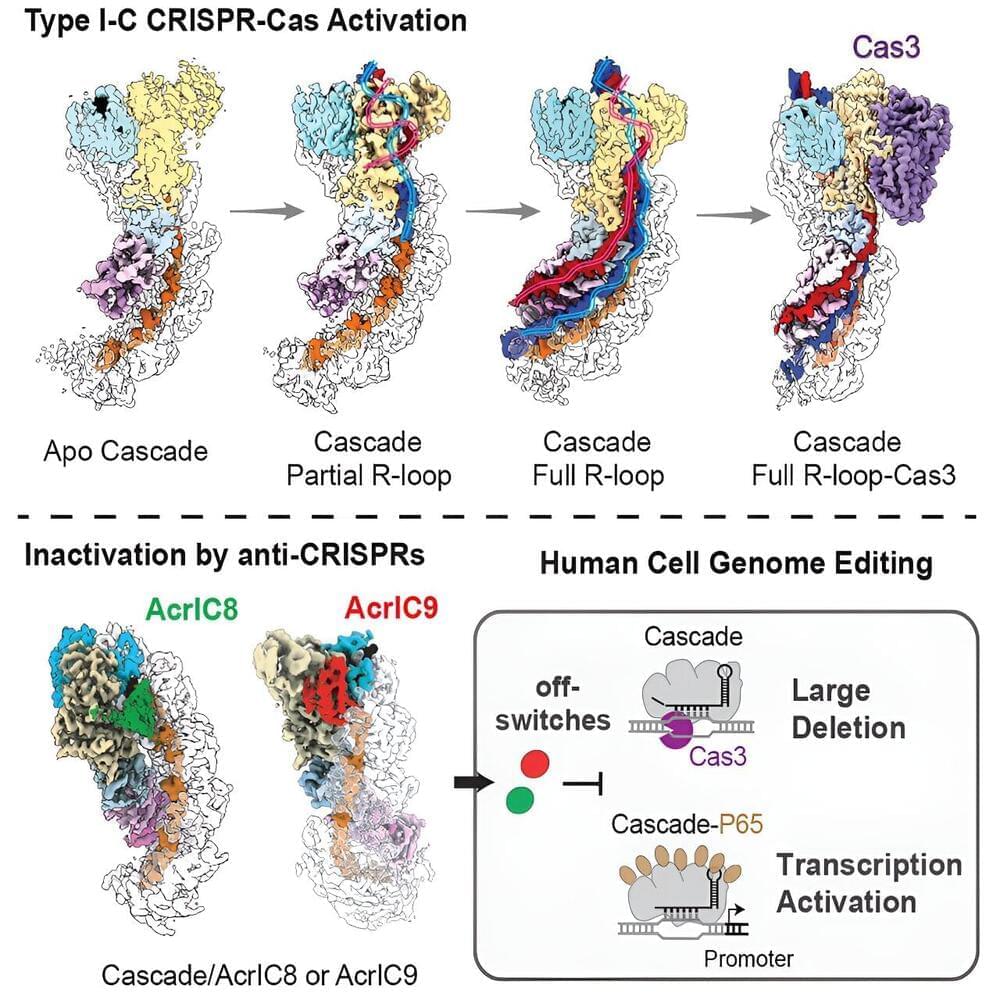
However, a newer and unique platform with the potential to make large-sized DNA removals, called Type I CRISPR or CRISPR-Cas3, waits in the wings for potential therapeutic use.
A new study from Yan Zhang, Ph.D., Assistant Professor in the Department of Biological Chemistry at the University of Michigan Medical School, and her collaborators at Cornell University develops off-switches useful for improving the safety of the Type I-C/Cas3 gene editor. The study, “Exploiting Activation and Inactivation Mechanisms in Type I-C CRISPR-Cas3 for 3 Genome Editing Applications,” is published in the journal Molecular Cell.