A new paper in Nature Communications illuminates how a previously poorly understood enzyme works in the cell. Many diseases are tied to chronic cellular stress, and UMBC’s Aaron T. Smith and colleagues discovered that this enzyme plays an important role in the cellular stress response. Better understanding how this enzyme functions and is controlled could lead to the discovery of new therapeutic targets for these diseases.
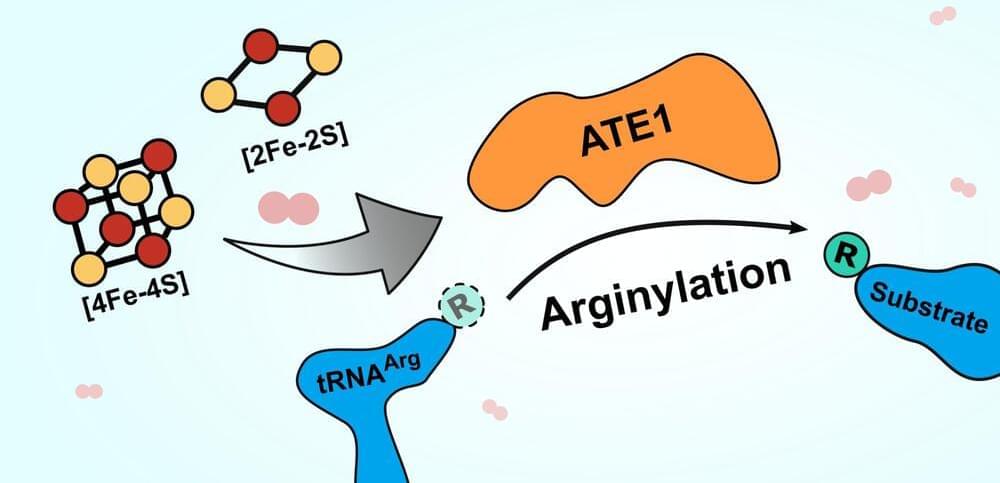
The enzyme is named ATE1, and it belongs to a family of enzymes called arginyl-tRNA transferases. These enzymes add arginine (an amino acid) to proteins, which often flags the proteins for destruction in the cell. Destroying proteins that are misfolded, often as a result of cellular stress, is important to prevent those proteins from wreaking havoc with cellular function. An accumulation of malfunctioning proteins can cause serious problems in the body, leading to diseases like Alzheimer’s or cancer, so being able to get rid of these proteins efficiently is key to long-term health.
The new paper demonstrates that ATE1 binds to clusters of iron and sulfur ions, and that the enzyme’s activity increases two-to three-fold when it is bound to one of these iron-sulfur clusters. What’s more, when the researchers blocked cells’ ability to produce the clusters, ATE1 activity decreased dramatically. They also found that ATE1 is highly sensitive to oxygen, which they believe relates to its role in moderating the cell’s stress response through a process known as oxidative stress.