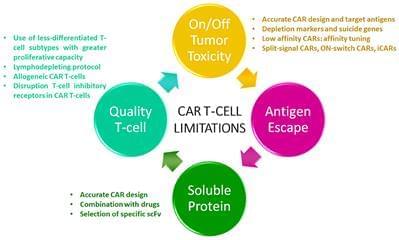
Multiple myeloma (MM) remains an incurable disease regardless of recent advances in the field. Therefore, a substantial unmet need exists to treat patients with relapsed/refractory myeloma. The use of novel agents such as daratumumab, elotuzumab, carfilzomib, or pomalidomide, among others, usually cannot completely eradicate myeloma cells. Although these new drugs have had a significant impact on the prognosis of MM patients, the vast majority ultimately become refractory or can no longer be treated due to toxicity of prior treatment, and thus succumb to the disease. Cellular therapies represent a novel approach with a unique mechanism of action against myeloma with the potential to defeat drug resistance and achieve long-term remissions. Genetic modification of cells to express a novel receptor with tumor antigen specificity is currently being explored in myeloma. Chimeric antigen receptor gene-modified T-cells (CAR T-cells) have shown to be the most promising approach so far. CAR T-cells have shown to induce durable complete remissions in other advanced hematologic malignancies like acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). With this background, significant efforts are underway to develop CAR-based therapies for MM. Currently, several antigen targets, including CD138, CD19, immunoglobulin kappa (Ig-Kappa) and B-cell maturation antigen (BCMA), are being used in clinical trials to treat myeloma patients. Some of these trials have shown promising results, especially in terms of response rates. However, the absence of a plateau is observed in most studies which correlates with the absence of durable remissions. Therefore, several potential limitations such as lack of effectiveness, off-tumor toxicities, and antigen loss or interference with soluble proteins could hamper the efficacy of CAR T-cells in myeloma. In this review, we will focus on clinical outcomes reported with CAR T-cells in myeloma, as well as on CAR T-cell limitations and how to overcome them with next generation of CAR T-cells.
Multiple myeloma (MM) is an hematological malignancy characterized by the clonal proliferation of malignant plasma cells. Myeloma develops from a pre-malignant monoclonal proliferation of plasma cells (monoclonal gammopathy of undetermined significance) which progresses to smoldering myeloma and finally to symptomatic disease (1, 2). With an incidence of 5.6 cases per 100.000 people/year in Western countries it accounts for 1% of all cancers and around 10% of hematological malignancies. Diagnosis of MM is based on the presence of clonal plasma cells plus monoclonal protein in serum or urine and clinical manifestations including hypercalcemia, renal impairment, anemia and/or bone lesions (acronym: CRAB) (4, 5).








Comments are closed.