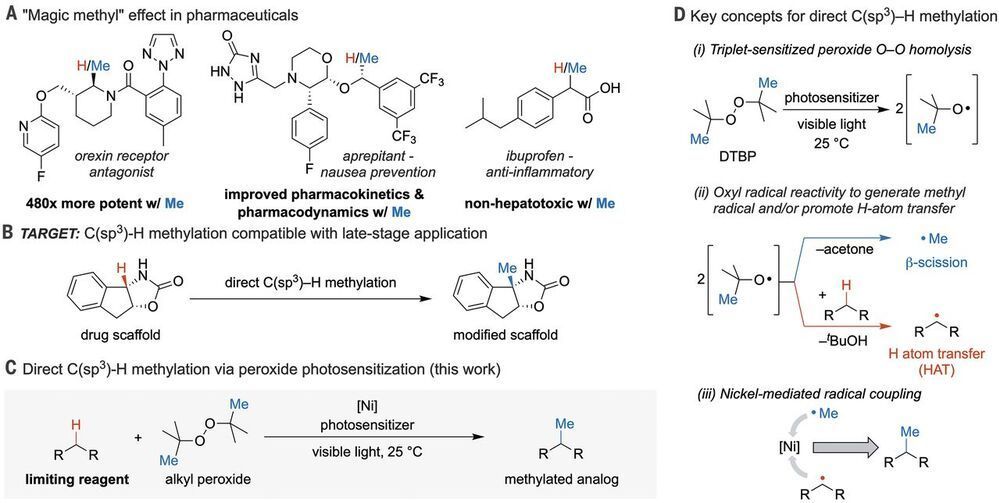
In pharmaceutical research, swapping out hydrogens for methyl groups is a frequent strategy to optimize small-molecule properties. Vasilopoulos et al. report a versatile, convenient, and comparatively safe method for methylation of carbon centers adjacent to nitrogen or aryl rings. Under carefully optimized conditions, di-tert-butyl peroxide plays a dual role as oxidant and methyl source. Cleaving the O–O bond through photosensitization produces butoxyl radicals, some of which cleave substrate C–H bonds, whereas others release methyl radicals that a nickel catalyst delivers to those activated substrates.
Science, this issue p. [398][1]
The “magic methyl” effect describes the change in potency, selectivity, and/or metabolic stability of a drug candidate associated with addition of a single methyl group.