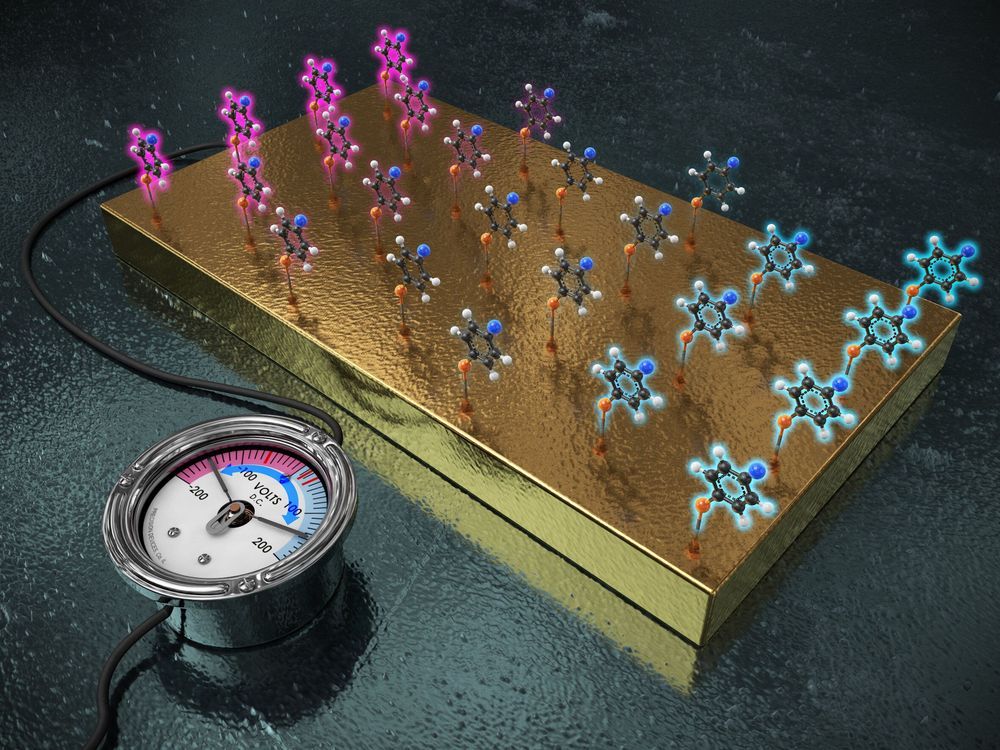
A team of researchers affiliated with several institutions in the Republic of Korea has found that it is possible to replace chemical functional groups with a gold electrode to control the reactivity of a molecule. In their paper published in the journal Science, the group describes attaching target molecules to a gold electrode to change the properties of immobilized molecules and how their technique performed when used to rate changes in the hydrolysis of certain esters.
In chemistry, functional groups are assortments of atoms that together work to attach carbon skeletons in organic molecules. All organic molecules have their own unique functional groups, which play an important role in the formation of molecules. Functional groups can also donate or take away electrons when one molecule comes into contact with another, which is how many chemical reactions occur.
Chemists have found that they can tinker with functional groups to speed up or slow down reactions to suit their needs, and because of that, functional groups play an important role in chemical synthesis. Unfortunately, developing reactions to produce desired products using functional groups has proven to be slow and difficult work. In this new effort, the researchers have found a way to replace the use of functional groups with a gold electrode to make the work easier. They simply attached molecules to a gold electrode and turned on the electricity. The technique allowed for more control over reactions by varying the amount of electricity supplied to the electrode. In such a capacity, the electrode was able to work as a “universal functional group” to inhibit or propel reactions when the researchers manipulated the amount of electricity applied to the electrode.