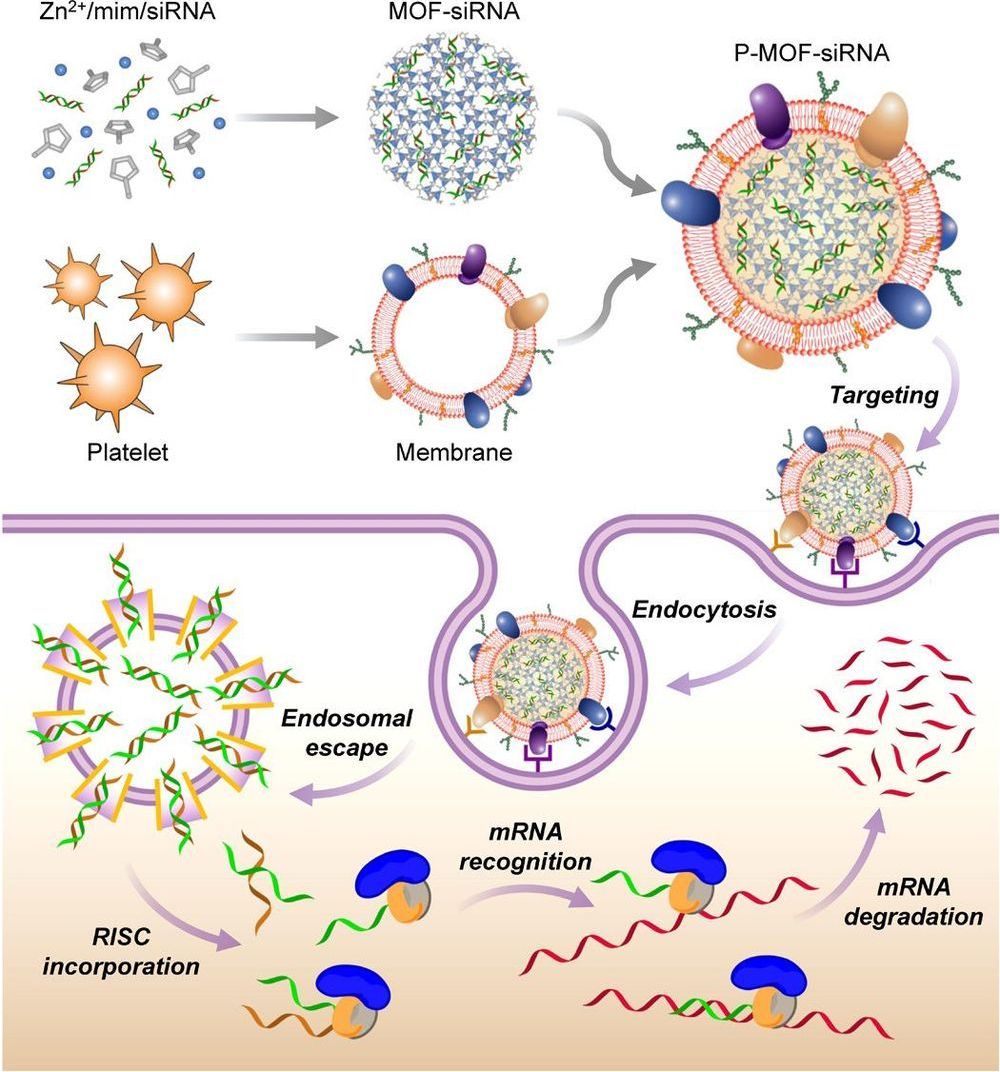
Small interfering RNA (siRNA) is a powerful tool for gene silencing that has been used for a wide range of biomedical applications, but there are many challenges facing its therapeutic use in vivo. Here, we report on a platelet cell membrane–coated metal-organic framework (MOF) nanodelivery platform for the targeted delivery of siRNA in vivo. The MOF core is capable of high loading yields, and its pH sensitivity enables endosomal disruption upon cellular uptake. The cell membrane coating provides a natural means of biointerfacing with disease substrates. It is shown that high silencing efficiency can be achieved in vitro against multiple target genes. Using a murine xenograft model, significant antitumor targeting and therapeutic efficacy are observed. Overall, the biomimetic nanodelivery system presented here provides an effective means of achieving gene silencing in vivo and could be used to expand the applicability of siRNA across a range of disease-relevant applications.
RNA interference (RNAi) is a naturally occurring mechanism for gene down-regulation that, since its first discovery in the late 1990s, has been widely leveraged as a tool for biological studies. Through a robust process mediated by the RNA-induced silencing complex present within the cytosol, target genes can be posttranscriptionally silenced via degradation of the corresponding mRNA. Small interfering RNAs (siRNAs) are short and well-defined double-stranded RNA molecules that can be synthetically manufactured to take advantage of the RNAi pathway. Over time, siRNAs have become an indispensable tool for validating gene function. They have also been widely explored as therapeutics for human disease , and an siRNA-based treatment for transthyretin-mediated amyloidosis was recently approved by the U.S. Food and Drug Administration.