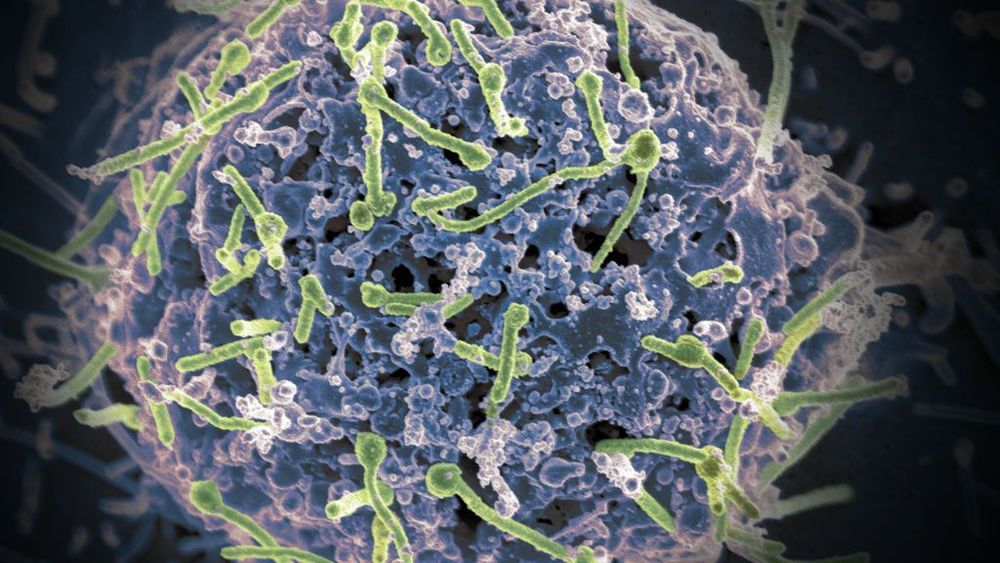
After more than two decades of research, the world finally has an approved Ebola vaccine.
The European Commission granted marketing authorization to Merck’s vaccine, known as Ervebo, on Monday, less than a month after the European Medicines Agency recommended it be licensed. It is currently being used in the Democratic Republic of the Congo under a “compassionate use” or research protocol similar to a clinical trial.
“The European Commission’s marketing authorization of Ervebo is the result of an unprecedented collaboration for which the entire world should be proud,” Ken Frazier, Merck’s chairman and chief executive officer, said in a statement.









Comments are closed.