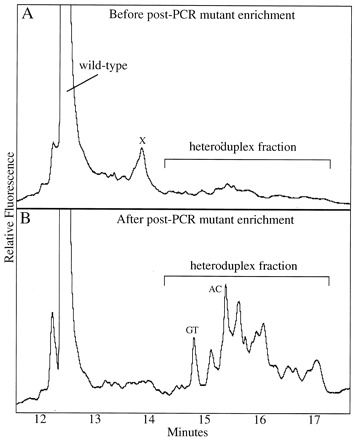
Knowledge of the kinds and numbers of nuclear point mutations in human tissues is essential to the understanding of the mutation mechanisms underlying genetic diseases. However, nuclear point mutant fractions in normal humans are so low that few methods exist to measure them. We have now developed a means to scan for point mutations in 100 bp nuclear single copy sequences at mutant fractions as low as 10–6.Beginning with about 10 human cells we first enrich for the desired nuclear sequence 10 000-fold from the genomic DNA by sequence-specific hybridization coupled with a biotin–streptavidin capture system. We next enrich for rare mutant sequences 100-fold against the wild-type sequence by wide bore constant denaturant capillary electrophoresis (CDCE). The mutant-enriched sample is subsequently amplified by high fidelity PCR using fluorescein-labeled primers. Amplified mutant sequences are further enriched via two rounds of CDCE coupled with high fidelity PCR. Individual mutants, seen as distinct peaks on CDCE, are then isolated and sequenced. We have tested this approach by measuring N-methyl–N ′-nitro–N-nitrosoguanidine (MNNG)-induced point mutations in a 121 bp sequence of the adenomatous polyposis coli gene (APC) in human lymphoblastoid MT1 cells. Twelve different MNNG-induced GC→AT transitions were reproducibly observed in MNNG-treated cells at mutant fractions between 2 × 10–6 and 9 × 10–6. The sensitivity of this approach was limited by the fidelity of Pfu DNA polymerase, which created 14 different GC→TA transversions at a mutant fraction equivalent to ~10–6 in the original samples. The approach described herein should be general for all DNA sequences suitable for CDCE analysis. Its sensitivity and capacity would permit detection of stem cell mutations in tissue sectors consisting of ~10 cells.